Our gut or digestive tract or more technically gastrointestinal (GI) tract is the gatekeeper to how foods get digested and absorbed into our body.
The GI tract is also responsible for excreting unwanted substances from the body through the colon.
You may have heard of the phrase ‘You are what you eat’. There is a lot of truth to this statement, as the foods we eat are the sources of nutrients required by our body for energy, growth, development, regeneration and repair and a whole range of other metabolic processes essential for life and wellbeing.
However, this phrase by itself does not show the entire picture. I would rephrase it to ‘You are what you eat, digest, absorb and metabolize’.
Whatever we ingest through our mouth has to be properly digested and absorbed before nutrients can be utilized by our body. In addition, how our body utilizes the absorbed nutrients is highly dependent on the functioning of various metabolic processes. For example, certain food compounds may cause immune reactivity, inflammation or allergic reactions to some individuals but not to others.
Dysfunction of the GI tract affects digestion, absorption and elimination. Some common GI disorders include:
- GI discomforts (e.g., bloating, flatulence, belching, heartburn, cramping)
- Maldigestion and malabsorption
- Diarrhea
- Constipation
- Gastric ulcers
- Irritable bowel syndrome (IBS)
- Irritable bowel disease (IBD)
- Diverticulitis
- …
In addition, because of the intimate relationship between the GI tract and other body systems, GI dysfunction can also contribute to disorders of other body functions including:
- Impaired immune function
- Sleep disorders
- Food sensitivity/intolerance/allergy
- Autoimmune disorders
- Metabolic diseases and disorders including diabetes and heart diseases
- Impaired brain and nervous system functions
GI dysfunction is also a major hidden stressor to the body, contributing to hypothalamus-pituitary-adrenal (HPA) axis dysfunction and cortisol dysregulation such as adrenal fatigue, and triggering cascading effects into the whole body (see Why Stress is the Culprit?). Therefore, GI dysfunction can be the underlying cause to many health challenges.
See Figure 1 below on the cascading effects of GI dysfunction.
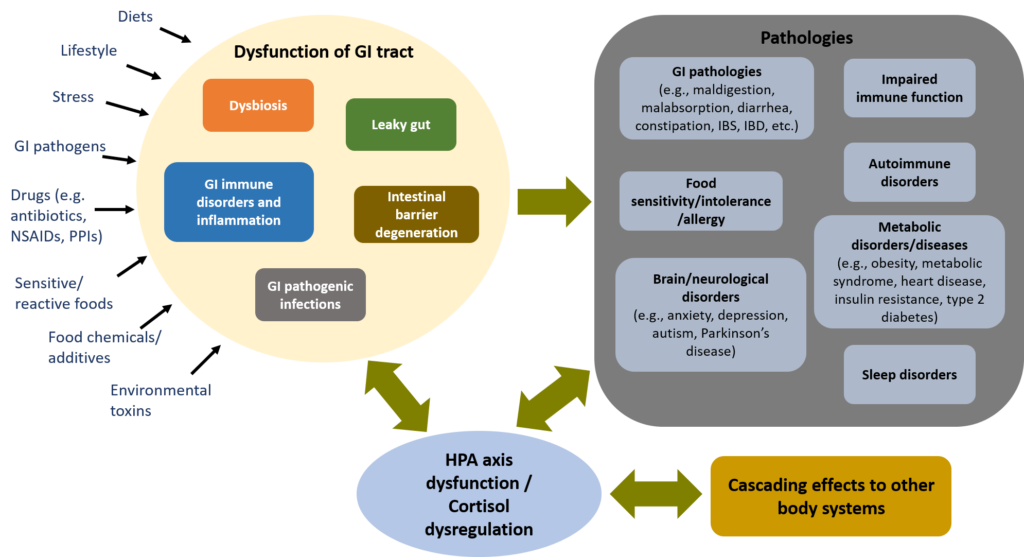
Figure 1. GI dysfunction: a major hidden stressor, affecting the whole body
How Our Gut Health Affects Our Immune Health
The GI tract is a long, continuous muscular tube that runs from the mouth, to the pharynx, the esophagus, the stomach, the small intestine, the large intestine, and the anus.
In addition to the skin, the internal lining of the GI tract, called the mucosal barrier, is the first line of defense between the external environment and the internal environment of the body.
In addition to providing a physical barrier, the mucosal barrier produces protective chemicals including stomach acid, enzymes, peptides, and antibodies that inhibit or destroy microorganisms.
The immune tissue in the mucosal barrier, called gut-associated lymphoid tissue (GALT) is found most abundantly in tonsils around the pharynx, in the lower portion of the small intestine and in the appendix. Adaptive immune response of GALT destroys or neutralizes pathogens, preventing them from breaching the mucosal barrier1.
GALT represent almost 70% of the entire immune system of the body. In addition, about 80% of immune cells (i.e., plasma cells) producing secretory IgA antibodies reside in GALT2. Therefore, the health of the GI mucosal barrier is crucial for the overall immune health.
Our Gut Flora: Friends or Foes?
The gut flora, or more technically gut microbiota, is an ecological community of commensal, symbiotic and pathogenic microorganisms found in the GI tract. These microorganisms include bacteria, fungi, viruses, archaea, and protists.
There is an estimated 10 to 100 trillion microbes reside in the intestinal track, outnumbering the number of cells in the human body. In addition, microbiome contains approximately 100x more genes than the human genome3,4.
Symbiotic microbiota plays important roles in the health of human host, including4,5:
- Digestion and metabolism of carbohydrates and proteins
- Fat/lipid metabolism that may have implication on fat storage and obesity
- Synthesis of essential vitamins including B vitamins and vitamin K
- Immunologic effects including regulation of anti-inflammatory and proinflammatory response and mediation of adaptive immune response
- Protection of GI tract from pathogen colonization; nourishment and regeneration of intestinal mucosal barrier
- Modulation of the human host’s behavior
It is important to have a diverse and healthy composition of gut microbiota. Dysbiosis or imbalance of gut microbiota, characterized by under-representation of symbiotic and commensal microorganisms and overgrowth of pathogenic microorganisms, has been associated with a number chronic diseases or disorders including5,6:
- Obesity
- Metabolic syndrome
- Nonalcoholic fatty liver disease
- Nonalcoholic steatohepatitis
- Type 2 diabetes
- IBS
- IBD
- Colorectal cancer
Our gut microbiota is influenced by the mode of delivery during childbirth, infant feeding mode (breast fed or formula fed), age, diet, lifestyle and stress. Exposure to antibiotics, whether prescribed or in the food chain of animal food products can also have profound and long-lasting detrimental effects on the microbiota5.
Leaky Gut: The Possible Culprit to Food Sensitivity/Intolerance and Autoimmune Disorders
Most of the digestion and absorption processes occur in the small intestine.
The mucosal barrier in the small intestine is a selective permeability barrier or gate-keeper that allows wanted substances such as digested nutrients to pass through, while prohibiting unwanted substances such as undigested food compounds, toxins, and pathogens to pass through.
The single layer of cells called enterocytes that line the surface of the small intestinal mucosal barrier provide the selective permeability function. Enterocytes are held together by intercellular adhesion called tight junction (TJ).
Leaky gut or intestinal hyperpermeability is a condition wherein degeneration of enterocytes or degeneration of the TJ result in dysregulation of permeability across enterocytes and gaps between enterocytes. See Figure 2 below.
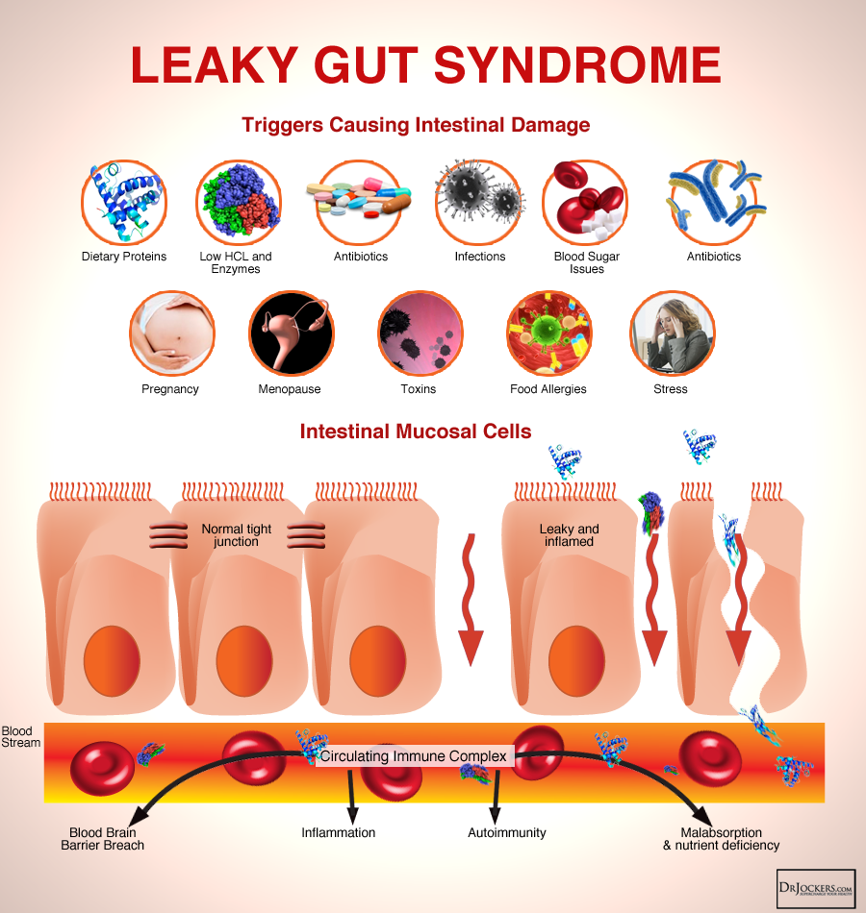
Figure 2. Leaky Gut Syndrome
Factors that may contribute to the disruption of the intestinal mucosal barrier, thus resulting in leaky gut include9–12:
- Diets low in fiber, high in refined sugar and fructose, and high in saturated fats
- Diets high in processed foods
- Gluten consumption
- Toxins in food including pesticides, herbicides, food additives and chemicals
- GI pathogenic infection
- Dysbiosis
- Vitamins A and D deficiency
- Alcohol consumption
- Certain drugs including antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs)
- Food sensitivity, which can both be a consequence and promoter of mucosal inflammation and leaky gut
- Stress
As mucosal barrier degenerates, both digestion and absorption of nutrients are affected.
As the gut lining becomes ‘leaky’, unwanted substances passes through. A growing number of researches although mostly preclinical have shown the correlation between leaky gut and immune function disorders. Leaky gut affects immune function in several ways as listed below.
Chronic Inflammation of the Mucosal Barrier
The unwanted substances that pass through the leaky barrier (e.g., undigested food compounds, toxins, and pathogens) can induce hyperactivation of the mucosal immune system (i.e., GALT), resulting in chronic inflammation that further compromises the integrity of the mucosal barrier7.
A vicious cycle can therefore develop.
Systemic Inflammation, Food Sensitivities/Intolerance and Food Allergies
The unwanted substances can also enter the bloodstream and trigger systemic immune response and hyperactivation, resulting in systemic inflammation in different parts of the body. Some symptoms associated with systemic inflammation may include:
- Chronic pain including sore, stiff or painful joints and muscle
- Chronic fatigue
- Skin issues such as hives, rashes, eczema, acne
When the immune system becomes hypersensitized to certain food compounds, food sensitivities or intolerance ensue.
Autoimmune Disorders
Foreign antigens from pathogens and food proteins that leak into the bloodstream can have molecular mimicry to self-antigens of specific tissues in the body, thus may sensitize immune response against self-antigens.
This may contribute to autoimmune disorders such as type 1 diabetes, celiac disease, systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis, wherein immune system attacks and causes destruction to own body’s tissues and organs8,9.
Gut and Brain Health: A Crucial Link That Cannot Be Ignored
The interconnection and interaction between the gut and the brain, called the gut-brain axis, has received a lot of attention in neuroscience research (see Figure 3).
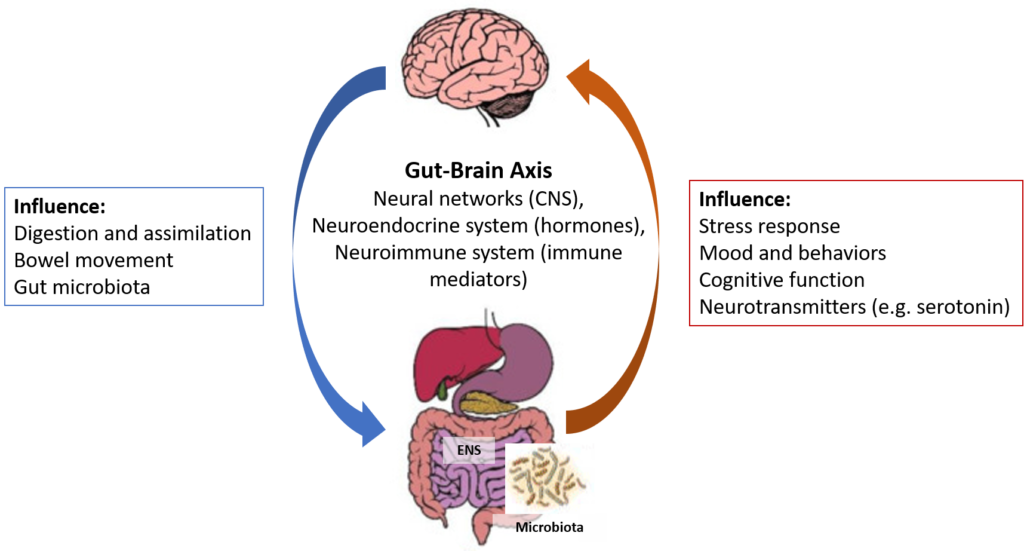
Figure 3. The Microbiota-Gut-Brain Axis
The gut-brain axis represents a functional relationship between the GI tract and the brain/central nervous system (CNS). This axis is a bidirectional biochemical signaling between the GI tract and the brain/CNS through the neural network, the neuroendocrine system, the neuroimmune system and the metabolic system.
The gut is often considered as the second brain, with its own enteric nervous system (ENS) that intrinsically controls GI activity. The ENS communicates with the CNS through the vagus nerve.
The linkage between the gut and the emotion and cognitive function of the brain has long been recognized in terms of regulation of HPA axis and stress-related responses13.
An emerging and growing field of neuroscience research is the involvement of gut microbiota as a critical component of the gut-brain axis, i.e. the microbiota-gut-brain axis. Exposure to psychological stress, even short-term, can alter the gut microbiota and vice versa where alteration of gut microbiota can influence stress responsiveness, stress-related behavior, and set-point for HPA axis activation13.
Preclinical studies and initial clinical studies have shown the intriguing and complex interaction and correlation among gut microbiota, leaky gut, and cognitive/psychiatric/neurological disorders such as autism spectrum disorders, anxiety, depression, schizophrenia, and Parkinson’s disease14–16.
Sleep Disorders: Gut and Melatonin (The Sleep Hormone)
Melatonin, often known as the sleep hormone, regulates our sleep-wake cycles.
The production of melatonin by the pineal gland in the brain is regulated by the normal light/dark conditions. The pineal gland secretes melatonin at night to prepare the body to go to sleep, while the secretion of melatonin is suppressed by day light.
Melatonin is not just a sleep hormone, it is also an important regulator of many biological functions including GI functions (e.g., food intake, digestion, GI motility, inter-organ communication between liver and gut), reproductive function, immune function, and antioxidant and anti-inflammatory defenses17,18.
In addition to the pineal gland, the enterochromaffin (EC) cells in the GI mucosal barrier also produce melatonin. The GI tract produces 400 times more melatonin than the pineal gland17,18. As opposed to the pineal gland, the production of melatonin by the gut is not governed by the circadian rhythm.
Therefore, GI dysfunction may compromise melatonin production, thus affecting sleep and other metabolic functions regulated by melatonin.
Summary: What Contributes to GI Dysfunction?
GI dysfunction is a major stressor that contributes to HPA axis dysfunction and cortisol dysregulation, and has widespread effects on many body functions. It is therefore crucial to maintain good GI health.

Related Articles
References
- Marieb E, Hoehn K. Human Anatomy & Physiology. 10th ed. San Francisco: Pearson Benjamin Cummings; 2016.
- Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153 Suppl 1(Suppl 1):3-6.
- Bianconi E, Piovesan A, Facchin F et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40(6):463-471. doi:10.3109/03014460.2013.807878
- Morowitz MJ, Carlisle EM, Alverdy JC. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg Clin North Am. 2011;91(4):771-85, viii.
- Quigley EMM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y). 2013;9(9):560-9.
- Mirjana R. Function of the microbiota. Best Pract Res Clin Gastroenterol. 2013;27(1):5-16. doi:10.1016/j.bpg.2013.03.006
- Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017;10(2):307-317.
- Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol. 2012;42(1):102-11.
- Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. Published 2017 May 23. doi:10.3389/fimmu.2017.00598
- Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7(3):1565-76. Published 2015 Feb 27. doi:10.3390/nu7031565
- König J, Wells J, Cani PD, et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7(10):e196. Published 2016 Oct 20. doi:10.1038/ctg.2016.54
- Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. Published 2014 Nov 18. doi:10.1186/s12876-014-0189-7
- Foster J, Rinaman L, Cryan J. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7. doi:10.1016/j.ynstr.2017.03.001
- Julio-Pieper M, Bravo J, Aliaga E, Gotteland M. Review article: intestinal barrier dysfunction and central nervous system disorders – a controversial association. Aliment Pharmacol Ther. 2014;40(10):1187-1201. doi:10.1111/apt.12950
- Mayer E, Tillisch K, Gupta A. Gut/brain axis and the microbiota. Journal of Clinical Investigation. 2015;125(3):926-938. doi:10.1172/jci76304
- Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G. Gut Microbiota Dysfunction as Reliable Non-invasive Early Diagnostic Biomarkers in the Pathophysiology of Parkinson’s Disease: A Critical Review. J Neurogastroenterol Motil. 2018;24(1):30-42.
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011;17(34):3888-98.
- Claustrat B, Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015;61(2-3):77-84. doi:10.1016/j.neuchi.2015.03.002

